In the fast-evolving, highly regulated world of pharmaceutical communication, staying compliant while embracing digital transformation is a formidable challenge. The intricate web of laws, regulations, and codes—collectively known as rules—governs how pharmaceutical companies communicate with healthcare professionals (HCPs), the public, and patients. Notable examples include IFPMA, EFPIA, EU Directive 2001/83/EC, FDA Code of Federal Regulations Title 21, PhRMA Codes, WHO’s “Ethical Criteria for Medicinal Drug Promotion,” ABPI 2021 Code of Practice (UK), ENLI (DK), Code de la Santé Publique (FRA), and HWG (DE).
Interpretation Variability and Digital Challenges
Despite the similarities among these rules, their interpretation can vary significantly across different legal, regulatory, and compliance departments of pharmaceutical and biotech companies, as well as regulatory bodies and governmental institutions, both locally and internationally. The rapid pace of digital transformation has introduced unimaginable use cases when these codes were initially drafted. As a result, the digital realm often faces even greater interpretative variability than traditional channels, creating uncertainty within the risk-averse cultures of Marketing, Communication, and Medical departments in pharmaceutical companies. This uncertainty has led to establishing internal company practices in the absence of specific rules, which we refer to as common practices.
Omnichannel Strategies and Compliance
These are outlines of some typical ingredients of omnichannel strategies and executions, that can serve as a guide to consult on the dos and don’ts of existing rules for tactic development, and traffic driving, often in the context of product launches.
Public & Patient Communication
Prescription Product Content
The rules on marketing prescription products to the public are stringent, with a global consensus that such promotion is prohibited, except in the US and New Zealand. Patients prescribed a drug can receive non-promotional, educational content from pharmaceutical companies, typically in the form of patient leaflets or support programs aimed at improving treatment adherence and addressing the psycho-social impacts of chronic diseases. While rules do not mandate gating for this non-promotional content, companies often implement soft or hard gates to facilitate personalized communication and data collection for service improvement.
Disease Awareness Campaigns
Disease awareness campaigns are generally permitted and focus on providing general information on diseases and health management without promoting specific products. These campaigns should refrain from creating demand for a particular medicine, but mentioning treatment options such as therapeutic classes somewhat is often permissible in several jurisdictions.
HCP Communication
Prescription Product Content
It’s important to differentiate between promotional marketing and educational or disease-awareness content. Promotional content, usually made by commercial departments, must be thorough, objective, and match the approved Summary of Product Characteristics (SPC). All claims must be backed by peer-reviewed studies, and promotional activities can start once the product gets marketing authorization. However, medical departments can educate about investigational compounds up to phase 3 study results, with promotion starting after approval.
Disease Awareness Campaigns for HCP
Disease awareness campaigns for HCPs can link non-product content to promotional material if there is proper evidence and authorization. Problems can arise when linking non-branded content, like a LinkedIn ad, to disease awareness content containing promotional material because of concerns about accurately verifying and targeting HCPs.
Typical Omnichannel Use Cases and Compliance Questions
- Local Traffic to Global Websites Local traffic drivers can lead to global or regional product websites if the content complies with local regulations and appropriate gating is in place for local content.
- Patient Cases on Pharmaceutical Websites Non-branded disease awareness content can include patient cases, but promotional content must avoid subjective depictions. Some countries allow anonymized patient cases representative of clinical trial outcomes.
- Promoting Patient Support Programs Creating awareness of patient support programs through online banners is challenging due to controlling exposure to non-patients. Paid search is more controlled, allowing specific search terms to target interested patients.
- Disease Awareness Campaigns by Sole Authorization Holders Companies holding sole authorization for treatment must exercise caution in disease awareness campaigns to avoid indirectly promoting their product.
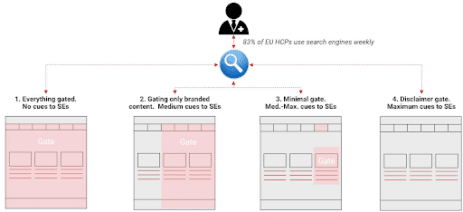
- Gating for HCP Sites Gating requirements for HCP sites vary significantly by country, with some requiring complex gates and others allowing soft gates.
- Use of Images in Promotional Materials. Images in promotional materials must be used cautiously to avoid misleading interpretations about the product’s efficacy.
- Jurisdiction Over Online Promotional Events. Jurisdiction for online events often follows the rules of the country where the organizing department is based. Still, international events may also apply traditional event rules.
- Linking Promotional and Medical Content. Digital platforms must ensure clear differentiation between promotional and medical content, with varied practices across companies.
- Social Media for Promotional Content. Social media is rarely used for promotional materials outside the US and New Zealand due to access control challenges. Closed groups and HCP-only platforms are alternatives.
- MLR Process for Disease Awareness. Disease awareness content does not require MLR process approval, but internal approval processes are still necessary.
- Trade Name URLs for HCP Websites. The use of trade name URLs varies by country. Some countries allow it under certain conditions, while others prohibit it. Standard practices include search engines not indexing them and gating for HCP access. Here are some examples from various national codes:
-
- Netherlands: The Foundation for the Code for Pharmaceutical Advertising allows trade name URLs.

-
- Norway: The Norwegian Association of the Pharmaceutical Industry states RX drug advertising can occur on HCP-only websites, implying these sites should be gated and not indexed by search engines.

-
- Ireland: The IPHA code permits using the trade name in the web address for HCP-targeted sites.

-
- Spain: It is prohibited to publish or share promotional content for prescription drugs openly. Trade name URLs are considered promotional, so they must not appear in non-HCP materials and should be gated and not indexed by search engines.

-
- UK: The MHRA Blue Code allows drug promotion on HCP websites with clear signposting and a softer gating requirement than other EU countries. Trade name URLs are common.

-
- Italy: The Farmindustria code does not specifically address trade name URLs. Trade name websites should not be indexed and must contain a gate. Many trade name URLs are registered but not activated.

-
- France: According to the “Charte pour la communication et la promotion des produits de santé,” the site must be gated if a domain name includes a prescription drug name. Trade name URLs are allowed for HCP sites with proper security measures.

Conclusion
These examples highlight the complexities and gray areas in pharmaceutical communication compliance. Vertic, now part of the Globant family, continuously develops its point of view and common practices based on its extensive experience with over 100 omnichannel projects in the healthcare industry. As digital transformation progresses, the combined forces of Vertic and Globant’s Healthcare and Life Sciences Studio enable us to stay informed and adapt to this evolving landscape.




