Let’s start from the beginning, formulating the most basic question: what is cancer and from where it comes?
Cancer is a genetic disease produced by specific mutations at the DNA level. It modifies the expression of our genes (those that define the human characteristics, or phenotype to be more technical). Due to this, it generates undesired and uncontrolled effects on the human body. For example, uncontrolled growth of cells, creating tumors whose purpose is only to grow and replicate itself.
Let’s back up a bit and examine how DNA can mutate and how that can guide the proliferation of a malign tumor.
Cell replication
You can think about DNA as a sequence of 3 billions of letters (A, T, C, G) that form patterns. Each pattern defines certain “functionality on our body.”
Every human being starts from a single cell, half DNA provided by the father and the other half by the mother, during the fertilization process. That single cell then has one full copy of the entire DNA. From a single cell, it ends up being a human adult with about 37 billion cells. How did that happen? What is the concept of grown from a molecular point of view?
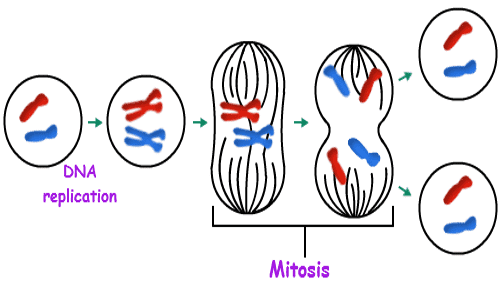
Each cell has a copy of the DNA. Its primary purpose is to replicate itself several times, copying its DNA on each replication (in a process called mitosis or meiosis, for a particular case). So starting with one cell, then it becomes two, two becomes 4, then 8, and so on, growing at an exponential level.
Throughout our life, the same cycle continually repeats over and over, having some cells replicating and some others dying as a natural process. The pace of replication is faster during our first quarter of life, and the pace of cells dying is faster in our last quarter.
DNA mutations
DNA is continually being copied from one cell to another, as we know. It goes on to explain that mutations take place when there is a mistake in copying DNA from one cell to another. There could be different reasons why a mutation takes place:
- A simple error on the copy process (less than 1 per 100,000,000 nucleotides)
- Environmental conditions, such as chemical exposure, radiation, ultra-violet rays, tobacco.
- Unknown reasons
There are different kind of mutations that can take place:
- Changing one letter by another
- Inserting/removing one letter
- Changing the letter-pattern that identifies the beginning/end of a gene region
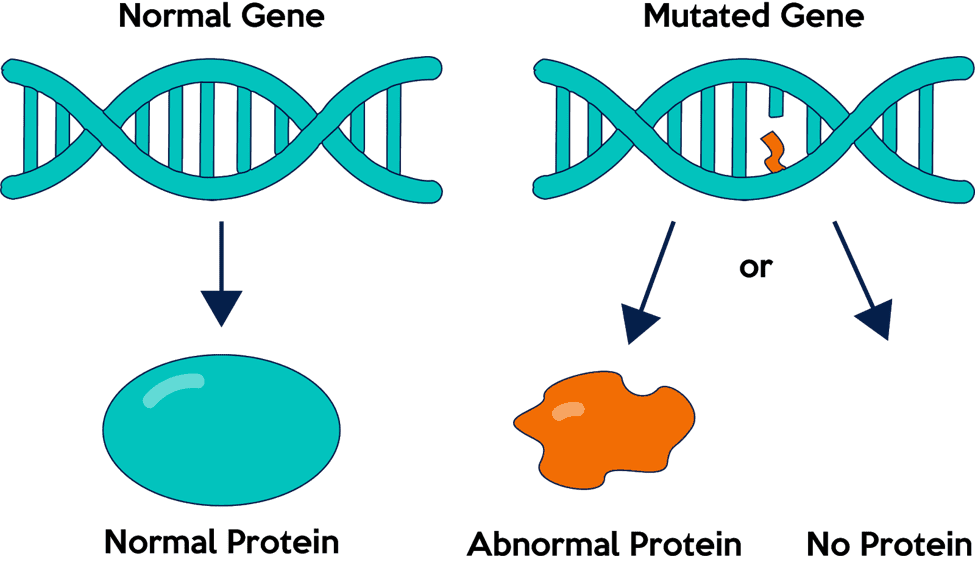
Each mutation can have different impacts (beneficial, harmful, or neutral). A harmful mutation taking place at a gene region can cause a malformation of it. The occurrence translates into a malfunctioning protein or the absence of such protein.
Development of cancer
We can now state that the development of cancer is a process of accumulated mutations during cell replication. It keeps on replicating until it finally reaches a mutated copy of our DNA to a new cell that has specific capabilities:
- Growth signal autonomy: the ability to proliferate even in the absence of a received growth signal
- Evasion of apoptosis: apoptosis refers to the programmed cell death. A cell evading this mechanism means that it lives longer than a normal cell.
- Insensitivity to antigrowth signal: this ability refers to the cell keep growing (replicating) even if the body is sending signals to stop it.
- Sustained angiogenesis: ability to send out signals to the cells of nearby blood vessels, inducing these them to grow extensions to form a supply chain and drainage channels for the growing tumor
- Limitless replicative potential: the ability of the cell to become immortal (indefinite growth and division)
- Capacity to invade tissue and growth at metastatic sites
Those capabilities all together give place to cells to grow indefinitely. It doesn’t die like other cells and ignores the suppressor mechanism to stop growing. It creates supply channels from nearby blood vessels to bring oxygen to the cancer cells. Finally, using its ability to replicate infinitely, those cancer cells can travel through the blood and infect other organs in a process called metastasis.
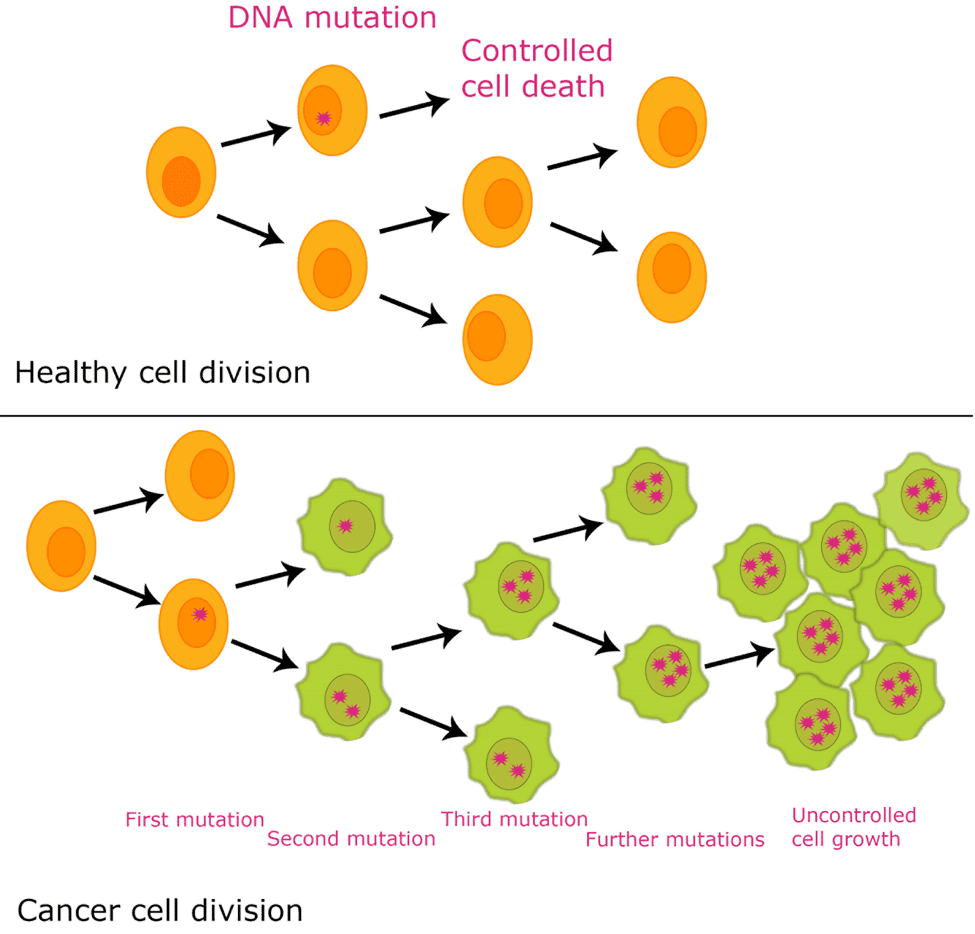
Since cancer is a disease generated by mutations on the DNA, the tumor cells can (and usually will) keep mutating. It leads to what is known as tumor diversity. It means having cells of the same tumor with different mutations of the DNAs. The cells react differently to drugs and have a different impact on the body, making it even more challenging to treat.
How can genomics help to fight this disease?
Genomics refers to the study of the genome, and the genome is the understanding of human DNA. The study helps us in identifying genes and their function and expression through proteins.
Since a series of mutations produce cancer, each cancer is different. There are not two of the same type. Hence, being able to identify each cancer particularity can help to guide a personalized treatment that better fits the person and disease.
Finding a cure for cancer means to change a patient’s DNA to revert it to a healthy state that doesn’t express the capabilities of tumor cells previously discussed. Let’s keep that area of active research aside and focus on detecting DNA changes that can lead to the disease.
Genomics analysis of tumors
Tumor cells are living small pieces of life. Therefore they carry a copy of DNA, a mutated version as we saw earlier. The first thing to do to start a genomics study of cancer is to get it sequenced. The process starts by taking a sample (it could come from a biopsy), use a sequencer device, and get; as a result, the DNA of the disease. Remember that tumors may be heterogeneous, so there could potentially be different DNAs within the same tumor, but let’s focus on the simple case.
Now is where the bioinformatician plays a vital role, doing things like:
- Compare sequenced DNA with a reference base (along with the tumor sample it is also taken a sample of blood or saliva, with healthy DNA), to detect with regions are the ones affected by the mutations
- The identified regions correlate with public genomic databases, to detect what kind of mutations took place and what genes/proteins were affected
- Knowing the regions impacted by the mutations, identify the biological consequences that affect the cells carrying that cell
- There could be enough evidence to make some prediction in regards to the disease development by knowing the biological impact.
Even though that may sound simple at first glance, each of those steps has taken years of research, and still, there are many unknowns, but the little we know opens a new angle and hope for this fight.
A crucial contribution from the genomics standpoint to early detect this disease is about detecting “driver mutations.” Drivers actively contribute to the growth of the tumor.
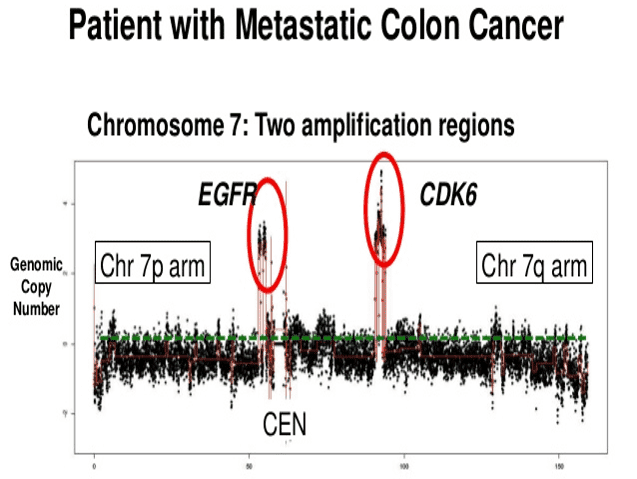
Image from “Genomics and personalized medicine” book.
In the image above, after sequencing the tumor, it was found increased copies of the EGFR (epidermal growth factor) gene. This gene has particular importance on cancer diagnosis since it is involved in the signaling pathways within the cell that promote cell growth and division (proliferation). Those critical genes involved in developing any of the tumor biological abilities listed above (see section “development of cancer”) are called oncogenes. They are usually the primary target of any cancer genetic analysis.
Back to the example from the picture, that patient was treated with an EGFR inhibitor (erlotinib) as part of his therapy to stop or at least slow down the uncontrolled growth and proliferation of tumor cells.
It is an excellent example of targeted therapy (personalized medicine in a broader concept) done thanks to the sequencing of the tumor cells and the use of genomics to find the regions affected.
Of course, there are many other approaches, tools, methods, and theories which are currently under research . Some of them under clinical trials to prove their efficiency in real patients. What explained here is just the tip of the iceberg, just enough to get a sense of on what direction the genomics research is going to.
What did we learn from cancer from a molecular level?
Having insights into the behavior of the disease in a molecular level gave scientists a few essential breakthroughs:
- Every tumor is different and has a different genomic profile
- Certain mutations are common in specific cancers and can indicate “signs” of early development
- Although there are many different types of cancer, the underlying molecular defects typically affect a handful of biological reactions (pathways), often involved in cell growth and proliferation.
- If specific mutations (called germline, between 5 and 20% of cancers) already took place on a person, they can pass from one generation to another. All cells of the baby carry those DNA mutations.
- A high-risk patient of developing the disease can alert other family members about the same risk.
Conclusion
There are tremendous efforts to be better equipped to fight against cancer. There are many angles and technologies; people around the globe are trying to make progress. Still, up to today, there is one truth that everyone agrees to and is (and will be for now) our first and primary weapon against this silence and deadly disease: prevention. Detecting cancer at an early stage can save your life.
Please read our article on potential breakthrough in cancer management by Machine Learning, to understand what the future of cancer treatment is.